Cloud vigilance efficiency
for life sciences
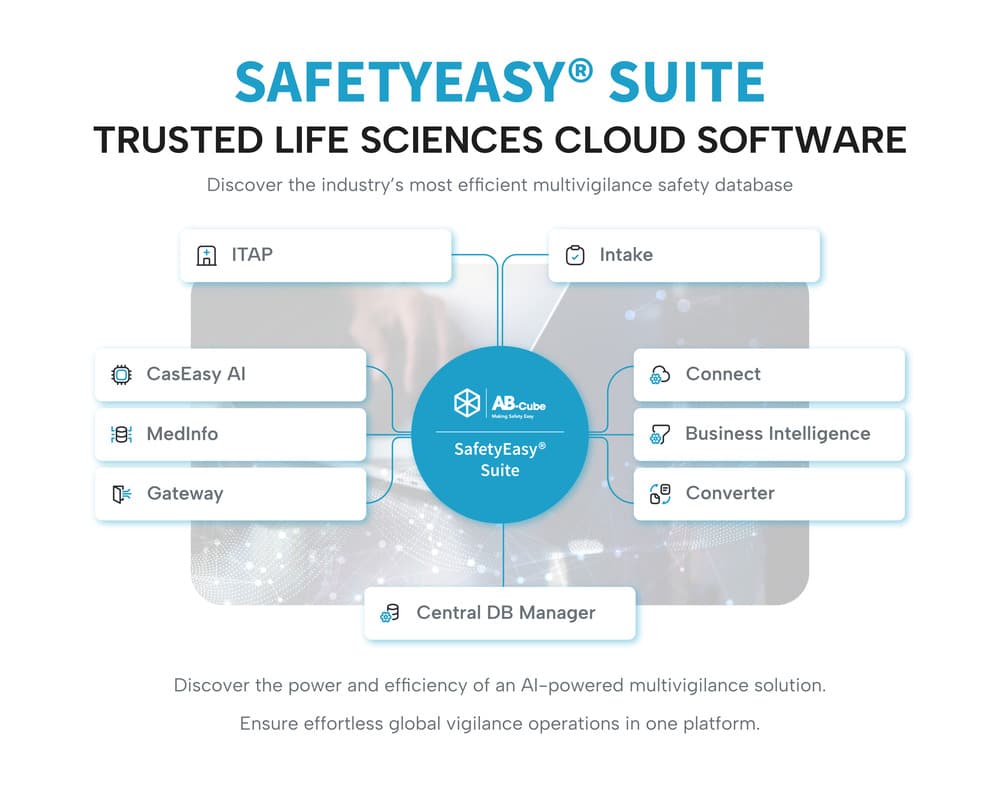
SafetyEasy® Suite delivers effortless global vigilance operations in a single AI-powered platform.
Empower your team with the most cost-effective cloud safety database software for pharmacovigilance compliance, medical device vigilance, cosmetovigilance and nutrivigilance.

Simplify & speed up case intake
Gain case creation efficiency
Enhance case analytics
Improve signal detection
Seamless system connectivity
Automate ICSR processing
MULTIVIGILANCE
Effortless compliance, smart automation
With GAMP5 and FDA CFR21 part 11 standards, SafetyEasy® means minimal additional validation effort for your safety team. Up-to-date validation dossiers and automatic system updates keep you aligned with the latest drug safety regulations.
Marketing Authorization Holders, pharmaceutical laboratories, CROs, pharmaceutical organizations and health authorities worldwide depend on agile, flexible and compliant SafetyEasy® multivigilance cloud software from AB Cube to optimize their safety processes.





Galaxy modules
Next-gen AI Galaxy modules
Reduce manual processes and enhance efficiency, compliance and signal detection with our next-AI generation Galaxy modules – available as add-ons to our industry-leading multivigilance management suite.
Our powerful automated case creation capabilities save your team time and effort. Generate cases from non-standard text with CasEasy AI and auto-extract form data with our OCR-driven Converter tool. Seamlessly integrate with any external system, connect safety data, and easily export to health agencies.
From optimized article screening with Literature Manager to easy AE reporting form creation with Webform, our Galaxy modules ensure fast, efficient and scalable compliance.

RECENT ARTICLES
News & events
Meet our experts and engage in a stimulating dialogue, gain some valuable insights from our webinars, grab a coffee at our exhibitor booth, participate in our endeavour of making drug safety easy.
New customer case study: PSI
“SafetyEasy® has become an essential tool, enabling us to efficiently deliver PV services to our clients. As a CRO we are taking advantage of SafetyEasy®’s onboarding flexibility, seamless upgrades...
New partnership with EvidentIQ
In our journey to make safety and clinical operations easier for our customers, we have already connected our safety platform to several EDC systems available on the market. Today, we are proud to announce a new partnership with our sister company EvidentIQ and its Marvin EDC system.
Upcoming: product launch webinar
We invite you to join us in December for an exclusive launch webinar where we will be unveiling a brand-new product that boosts the time-saving optimizations delivered by SafetyEasy® Suite – the industry’s most efficient multivigilance safety platform.


