SAFETYEASY® SUITE
Multivigilance
Harness the power of automation for your global regulatory requirements.
Align international vigilance operations teams with an intuitive, unified, cloud-based system trusted by industry leaders. SafetyEasy® Suite is a multivigilance management solution which integrates automation with existing drug safety processes of life sciences organisations to manage adverse event reporting and stay compliant with the latest regulations.
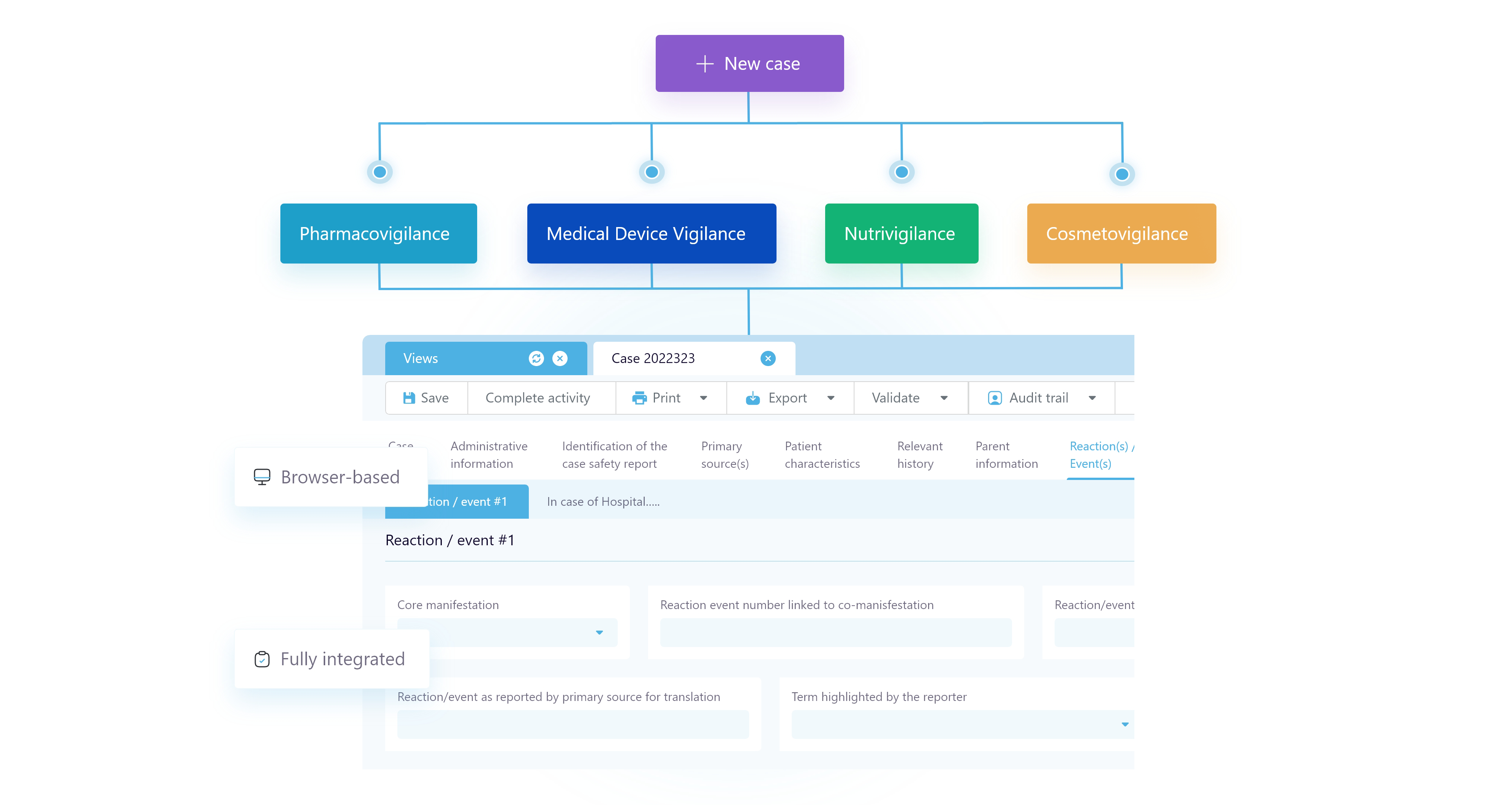
Features
Gain efficiencies with industry’s trusted cosmetics safety solution fully dedicated to the management of Adverse Events, always aligned with the best practice and modern regulations.
Report generation
- CIOMS I, MedWatch, …
- XML E2B (R2) and E2B (R3) HL7 Import/Export
- Line-Listing (PSUR, PBRER, DSUR, …)
- Summary tabulation (PSUR, PBRER, DSUR, ..)
- Automatic mail generation
- Signal detection: PPR, Qualitative
Tools
- MedDRA SMQ
- Integrated query module
- Duplicate check management
- Calendar (submission & follow-up management)
- Automatic mail generation
- Schedule and assign tasks
Data entry
- User-friendly data entry
- Inbuilt MedDRA codert
- Duplicate check management
- Generate narrative templates
- Follow-up management
- Attach documents
- E2B (R3) data entry
- E2B (R3) data entry import/export
- E2B (R2/R3) validation
- E2B (R2) import/export
Administration
- Full workflow management
- Users administration
- User’s group rights management
Integration
- AB Cube’s own Gateway or Web Trader integration
- Link with SafetyEasy® MI
- WHO Drug Dictionary (WHODD) integration
- XEVMPD/IDMP integration
- EudraCT link
Report generation
- CIOMS I, MedWatch, …
- XML E2B (R2) and E2B (R3) HL7 Import/Export
- Line-Listing (PSUR, PBRER, DSUR, …)
- Summary tabulation (PSUR, PBRER, DSUR, ..)
- Automatic mail generation
- Signal detection: PPR, Qualitative
Tools
- MedDRA SMQ
- Integrated query module
- Duplicate check management
- Calendar (submission & follow-up management)
- Automatic mail generation
- Schedule and assign tasks
Administration
- Full workflow management
- Users administration
- User’s group rights management
Integration
- AB Cube’s own Gateway or Web Trader integration
- Link with SafetyEasy® MI
- WHO Drug Dictionary (WHODD) integration
- XEVMPD/IDMP integration
- EudraCT link
Data entry
- User-friendly data entry
- Inbuilt MedDRA codert
- Duplicate check management
- Generate narrative templates
- Follow-up management
- Attach documents
- E2B (R3) data entry
- E2B (R3) data entry import/export
- E2B (R2/R3) validation
- E2B (R2) import/export
WE’RE READY
AI powered
AB Cube simplifies automation for your safety operations. SafetyEasy® Suite integrates seamlessly with your existing vigilance processes with minimal training. Leverage the power of automation with our 3-step process.
BASIC AUTOMATION
ROBOTIC PROCESS AUTOMATION
COGNITIVE AUTOMATION
Get ready to step into the world of pharmacovigilance powered by artificial intelligence.
Increased ROI on multivigilance operations
Industry’s first cloud-based pharmacovigilance software, SafetyEasy® was launched in 2006 when on-premise software was the norm. We accompany academic research centres, CROs and MAHs as their trusted safety software with our reliable technology.
Secure & compliant SAAS
HDS compliant server hosting for sensitive healthcare information, High Availability Infrastructure, Disaster Recovery Plan: Immediate fail over to distant server in case of disaster, HIPAA compliant, SSL web access, 99.99% SLA guaranteed, 21 CFR part 11 Audit Trail, ISO 27001 data center (Tiers III plus)
One-click submission
Smart E2B XML creator (R2, R3), multiple formats supported (MedWatch, CIOMS, Meddev, etc.) and email submission
Intuitive user interface
Automatically populated fields, narrative generation in just one click, smart MedDRA coding, integrated query module, fully customisable workflow
No hidden cost
Transparent terms and conditions since Day 1. Unlimited number of users, quarterly upgrades and support included in your license. Your support tickets would not circle the world, our support team based in France is promptly resolves bugs and queries
2 week implementation
Digitise your excel-based and paper-trail regulatory reporting with SafetyEasy® Suite in less than 2 weeks
Fully validated
GAMP 5 validation, online validation dossier available, validation dossier updated with each upgrade. Minimum validation effort required from the users
One-click submission
Smart E2B XML creator (R2, R3), multiple formats supported (MedWatch, CIOMS, Meddev, etc.) and email submission
No hidden cost
Transparent terms and conditions since Day 1. Unlimited number of users, quarterly upgrades and support included in your license. Your support tickets would not circle the world, our support team based in France is promptly resolves bugs and queries
Fully validated
GAMP 5 validation, online validation dossier available, validation dossier updated with each upgrade. Minimum validation effort required from the users

Boost Efficiency
- Manage cases with combination products
- Manage cases with products with different regulatory statuses depending of country
- Improve collaboration among PV teams in real-time with deadline and reminder management systems
- Edit reports in the blink of an eye without waiting for the system to load
- Get automatic system updates to stay current with the latest drug safety regulations

Powerful Reporting
Spontaneously generate submission ready regulatory reports automatically populated with entered data.
- CIOMS & MedWatch
- Periodic Safety Update Report (PSUR), Periodic Benefit-Risk Evaluation Report (PBRER), Development Safety Update Report (DSUR)
- Signal detection, line listings & summary tabulations
ALWAYS COMPLIANT
We’re making Multivigilance easy
Streamline safety operations on combination product reporting in different countries. Manage your PV and medical devices vigilance operations on a single application. Report biovigilance and pharmacovigilance adverse events on the same database for your FDA regulatory requirements.
Tackle the challenges of optimising operational efficiency while reporting sunscreen as a pharmaceutical product in FDA and a cosmetic product in EMA while processing safety cases. Tick one box to fulfil both requirements, pharmacovigilance as well as cosmetovigilance.
European & worldwide regulations compliance, FDA 21 CFR part 11 designed, SafetyEasy® Suite cloud-based multivigilance management solution conforms to FDA, EMA and MHRA requirements and is regularly upgraded to comply with latest regulations. Meeting combination product regulatory requirements was never easier.
NEWSLETTER
Sign up for latest news & resources news from AB Cube
FEATURES
Discover other vigilances
AB Cube fulfils vigilance requirements of every scale and scope, from prominent pharmaceuticals to emerging biotechnology firms. We provide drug safety and multivigilance solutions tailored to your needs.
Pharmacovigilance
Efficient drug safety operations with the expertise of industry veterans begins here
MedDevice Vigilance
Keep up with the changing regulations while optimising operation efficiency and
Cosmetovigilance
Benefit from the world’s first and only native cosmetics adverse events reporting system
Nutrivigilance
Ensure productive nutrient safety reporting with a native nutrivigilance solution